Leave Your Message
Cationic surfactants play a crucial role in various cleaning and conditioning applications due to their unique properties and functionalities. Unlike their anionic and nonionic counterparts, cationic surfactants possess a positive charge that enables them to interact effectively with negatively charged surfaces, such as dirt, oils, and hair. This characteristic makes them particularly valuable in formulations aimed at not only cleaning but also conditioning various materials, including textiles and hair.
The versatility of cationic surfactants allows them to be utilized in a myriad of products, from household cleaners to personal care items. Their ability to reduce surface tension and enhance foam stability leads to improved cleaning performance, while their conditioning properties help in softening and smoothing surfaces after cleaning processes. This introduction serves as a foundation for exploring the effective utilization of cationic surfactants, delving into their mechanisms, advantages, and best practices for incorporation in cleaning and conditioning applications. Emphasizing proper usage can lead to optimal results, whether in industrial settings or everyday household tasks.
Cationic surfactants are a unique class of surfactants characterized by their positively charged heads, which give them distinct properties and applications in cleaning and conditioning. These surfactants have a strong affinity for negatively charged surfaces, such as hair and skin, enabling them to effectively bind and provide conditioning benefits. This property makes cationic surfactants particularly valuable in personal care products, as they can improve the texture and manageability of hair while delivering moisture and reducing static.
In addition to their conditioning capabilities, cationic surfactants also exhibit excellent antimicrobial properties. This makes them effective agents for cleaning applications, especially in formulations aimed at sanitizing surfaces. Their ability to disrupt bacterial cell membranes contributes to their efficacy in eliminating unwanted microorganisms, ensuring cleanliness and hygiene. Furthermore, their low toxicity and biodegradability make them a suitable choice for environmentally conscious formulations, providing both performance and safety in various applications.
When selecting the right cationic surfactants for your cleaning needs, it is essential to consider the specific properties and functionalities required for effective performance. Cationic surfactants are particularly valued for their ability to provide positive charge, which enhances their adherence to negatively charged surfaces, allowing for superior cleaning and conditioning effects. Factors such as the intended application, the type of surfaces being cleaned, and the desired end-user experience play a crucial role in your choice.
For instance, if your focus is on fabric softening, look for cationic surfactants that offer excellent electrostatic charge properties and are gentle on fibers. Alternatively, for industrial cleaning applications, more robust cationic agents that can tackle heavy soils and grease may be necessary. It's also important to consider the compatibility of the surfactants with other formulation ingredients, such as anionic surfactants or polymers, to ensure stability and enhance overall cleaning efficacy. By carefully evaluating these aspects, you can select cationic surfactants that not only meet performance requirements but also align with environmental considerations and safety standards.
Cationic surfactants play a crucial role in enhancing the effectiveness of cleaning and conditioning formulations, particularly in personal care and household products. Their unique positively charged molecules enable them to interact with negatively charged surfaces, allowing for superior dirt and oil removal. According to a report by the Freedonia Group, the demand for cationic surfactants is projected to grow by 3.3% annually, reaching $1.5 billion by 2025. This highlights a significant market trend towards formulations incorporating these effective cleaning agents.
To effectively integrate cationic surfactants into formulations, one must consider both the concentration and the presence of co-surfactants. Studies indicate that a formulation containing 2-5% cationic surfactants can yield optimal cleaning results without compromising skin compatibility. Additionally, balancing the formulation with anionic or nonionic surfactants can enhance foaming properties and provide better overall performance. It is also advisable to utilize emulsifiers that can stabilize the mixture, improving consistency and longevity. Practical applications in hair conditioners and fabric softeners have consistently shown that formulations employing cationic surfactants lead to enhanced product efficacy, with users reporting improved texture and manageability in treated materials.
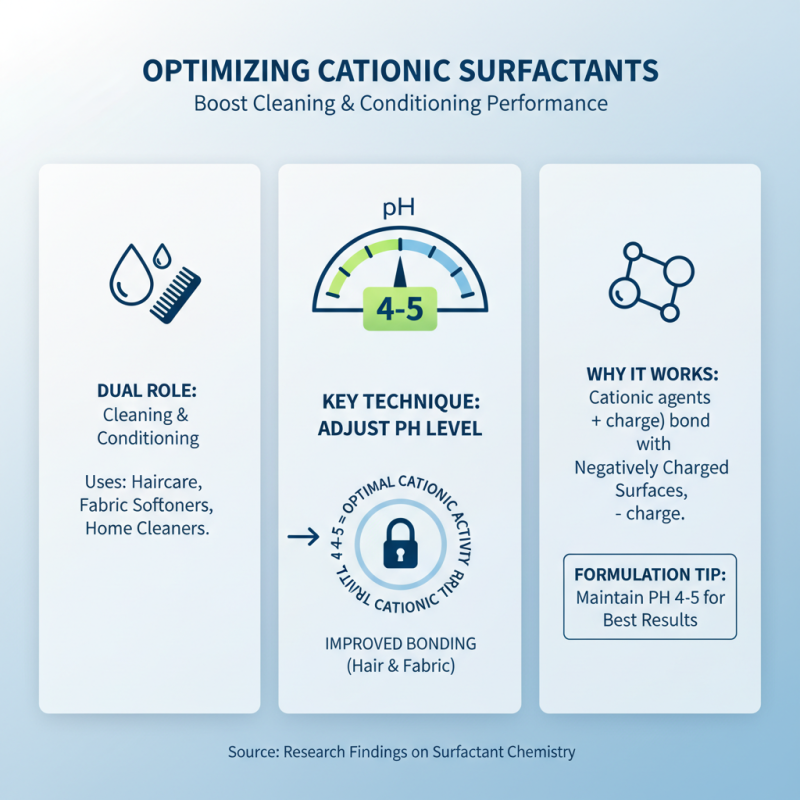
Cationic surfactants are widely recognized for their dual role in cleaning and conditioning, particularly in personal care and home cleaning products. To maximize their effectiveness, it is vital to adopt specific techniques during formulation and application. For instance, adjusting the pH level of the product can significantly enhance the performance of cationic surfactants. Research indicates that maintaining a pH value between 4 and 5 optimizes the availability of active cationic agents, thereby improving their bonding capability with negatively charged surfaces, such as hair fibers and fabric.
Another crucial technique is the utilization of co-surfactants. Data from the Journal of Surfactants and Detergents highlight that combining cationic surfactants with nonionic or amphoteric surfactants can lead to synergies that enhance cleaning efficacy without compromising the conditioning properties. This blend not only reduces surface tension more effectively than cationic surfactants alone but also allows for better penetration and adherence to contaminants. For example, experiments have shown that such formulations can increase the dirt and oil removal efficiency by up to 30%, making the cleaning process both effective and efficient.
Moreover, the application method can also impact the performance of cationic surfactants. Techniques such as incorporating them in a two-step cleaning process, where initial removal of dirt is followed by a conditioning step, can yield superior results. Studies suggest that this strategy allows for complete removal of contaminants while permitting the deposition of conditioning agents, thus optimizing the overall cleaning and conditioning effects of cationic surfactants in both industrial and household applications.
Cationic surfactants, widely used in cleaning and conditioning applications, have garnered attention for their efficacy but also raise safety and environmental considerations. According to the 2021 report from the Environmental Protection Agency (EPA), cationic surfactants are recognized for their antimicrobial properties, which contribute to their effectiveness in disinfecting surfaces. However, their potential toxicity to aquatic organisms necessitates stringent handling and disposal practices. Research indicates that certain cationic surfactants can significantly affect biodiversity, leading to toxic effects observed at concentrations as low as 10 mg/L. Users must therefore implement measures to mitigate environmental impact, such as utilizing biodegradable alternatives or ensuring proper dilution during use.
Moreover, the proper use of cationic surfactants must consider safety protocols to prevent adverse effects on human health. The American Conference of Governmental and Industrial Hygienists (ACGIH) highlights that prolonged skin exposure to concentrated cationic surfactants can cause irritation or allergic reactions. As such, protective equipment should be employed to safeguard workers during application. Additionally, industry guidelines recommend thorough training for personnel regarding the safe handling of these substances and the importance of following local regulations on chemical usage. By prioritizing these safety and environmental considerations, users can effectively harness the benefits of cationic surfactants while minimizing their risks.
