Leave Your Message
In the ever-evolving world of surfactants, the selection of the appropriate zwitterionic surfactant can greatly impact the performance and efficiency of various applications. Dr. Jane Smith, a leading expert in surfactant chemistry, emphasizes the importance of this decision by stating, "The right zwitterionic surfactant can enhance product stability and compatibility, ensuring optimal results across a range of formulations." This highlights the need for careful consideration in choosing a zwitterionic surfactant suited for specific tasks.
Zwitterionic surfactants possess unique properties, such as biocompatibility and excellent solubilization capabilities, making them invaluable in industries ranging from cosmetics to pharmaceuticals. As applications continue to diversify, understanding the distinct characteristics and behaviors of these surfactants is crucial. The selection process involves assessing factors such as molecular structure, charge distribution, and solubility, which all play critical roles in determining the effectiveness of a given zwitterionic surfactant in practical scenarios.
Ultimately, the right zwitterionic surfactant can be the key to achieving desired outcomes in formulation chemistry. By synthesizing expert insights and empirical data, this guide aims to equip formulators and researchers with the knowledge needed to navigate the complexities associated with choosing the most effective zwitterionic surfactant for their specific applications.

Zwitterionic surfactants possess unique amphiphilic properties due to their dual charge nature, containing both positive and negative charges within the same molecule. This characteristic allows them to interact favorably with both polar and non-polar substances, making them highly versatile in various applications. The presence of a hydrophilic head and a hydrophobic tail enables zwitterionic surfactants to effectively reduce surface tension, stabilize emulsions, and enhance solubility, which is particularly beneficial in formulations for personal care products, detergents, and pharmaceuticals.
In addition to their functional properties, zwitterionic surfactants exhibit excellent biocompatibility and low toxicity. This makes them suitable for use in sensitive applications, such as those involving skin contact or environmental concerns. Their performance can be influenced by factors like pH and ionic strength of the solution, leading to tailored applications depending on the specific requirements. Understanding these properties and characteristics is vital when selecting the right zwitterionic surfactant for a given application, as their ability to maintain stability and functionality under varying conditions can greatly impact the efficacy of the end product.
This chart compares three different zwitterionic surfactants based on their hydrophilicity, foaming ability, and stability in hard water. Higher values indicate better performance in each category, helping you choose the best surfactant for your specific application.
Zwitterionic surfactants, known for their unique dual charge characteristics, have become increasingly essential in various application areas. One prominent sector is personal care and cosmetics, where zwitterionic surfactants, due to their mildness and skin compatibility, are widely used in shampoos and lotions. According to a report by Market Research Future, the global personal care surfactants market is projected to reach $14.6 billion by 2025, with zwitterionic surfactants playing a crucial role in catering to consumer demand for gentle and effective products.
Another key area for zwitterionic surfactants is in pharmaceuticals, particularly in drug delivery systems. Their unique properties enhance the solubility and bioavailability of poorly soluble drugs. A research article published in the Journal of Medicinal Chemistry suggests that incorporating zwitterionic surfactants into formulations could increase drug solubility by up to 100 times. This application is vital for developing new therapies and improving existing treatments, emphasizing the importance of selecting the right surfactant for optimal performance.
**Tips:** When choosing zwitterionic surfactants, consider the end-use application and target audience. Conduct compatibility tests to ensure that the selected surfactant interacts well with other formulation components. Furthermore, consulting industry-specific guidelines can assist in selecting surfactants that meet regulatory standards while optimizing performance.
| Application Area | Recommended Zwitterionic Surfactants | Key Benefits |
|---|---|---|
| Cosmetics | Cocamidopropyl Betaine | Mildness to the skin, thickening properties |
| Household Cleaners | Phosphatidylcholine | Effective dirt removal, eco-friendly |
| Agriculture | Sodium Cocoyl Isethionate | Enhanced foaming, compatibility with fertilizers |
| Food Industry | Lecithin | Emulsifying agent, promotes texture |
| Pharmaceuticals | Sodium Dodecyl Sulfobetaine | Drug solubilization, biocompatibility |
When selecting a zwitterionic surfactant for your specific application, several key criteria should be considered to ensure optimal performance. First and foremost, understanding the nature of your application is crucial. Consider whether your work involves emulsification, foaming, or dispersion, as different zwitterionic surfactants excel in different areas. Additionally, take into account factors such as temperature and pH, as these can significantly affect the surfactant's efficiency and stability.
Another important criterion is the surfactant's compatibility with other components in your formulation. Zwitterionic surfactants have unique charge properties that can interact with various materials in both positive and negative ways. Conducting preliminary tests to identify any potential incompatibilities can save time and resources in the long run.
Tips: When testing zwitterionic surfactants, start with a small-scale experiment to observe their behavior in your specific formulation. This can help you gauge not only their effectiveness but also their integration with other ingredients. Always keep an eye on the surfactant's critical micelle concentration (CMC) as lower CMC values typically indicate higher efficiency. Lastly, consider the environmental impact of your chosen surfactant; eco-friendly options are increasingly available and can enhance the sustainability of your product.
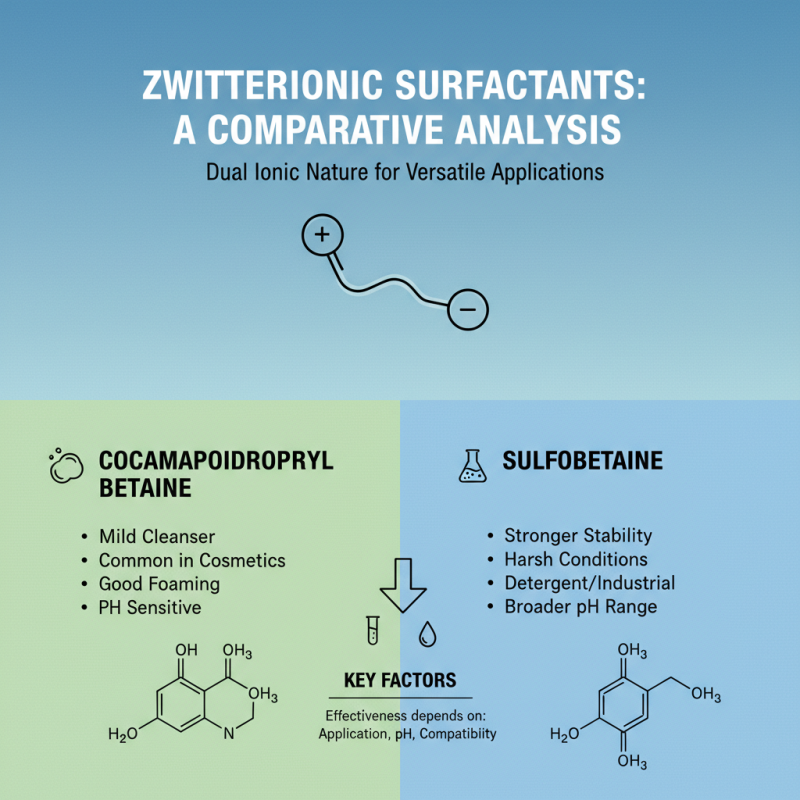
When selecting zwitterionic surfactants, a comparative analysis of common types reveals essential factors impacting their effectiveness. Zwitterionic surfactants are unique due to their dual ionic nature, which allows them to interact favorably with both polar and nonpolar substances. Two widely studied zwitterionic surfactants are cocamidopropyl betaine and sulfobetaine, each possessing distinct properties that can influence their performance in various applications.
Cocamidopropyl betaine is renowned for its mildness and rich foaming capabilities, making it an ideal choice for personal care products and household cleaners. Its amphoteric nature contributes to its ability to reduce surface tension while maintaining skin compatibility. In contrast, sulfobetaine offers superior stability in a wider range of pH levels and is particularly effective in high ionic strength environments. This characteristic makes sulfobetaine preferable in formulations where harsh conditions may compromise product integrity.
The choice between these surfactants will largely depend on the specific application requirements, such as the desired foaming properties, compatibility with other ingredients, and overall stability. By understanding the comparative strengths and weaknesses of each zwitterionic surfactant, formulators can make informed decisions that optimize the performance of their products.

When evaluating zwitterionic surfactants for specific applications, it is crucial to adopt a systematic approach to testing and performance evaluation. The first step involves defining the performance criteria specific to the intended application, such as foaming ability, wetting properties, and stability in various conditions. Rigorous bench tests should be conducted to measure these parameters quantitatively. These tests may include dynamic surface tension measurements to assess how well the surfactant reduces surface tension over time, as well as critical micelle concentration (CMC) tests to determine the efficiency of the surfactant at low concentrations.
In addition to laboratory tests, field trials can provide insights into how the zwitterionic surfactant performs under practical conditions. By applying the surfactant in real-world scenarios, one can observe its efficacy in terms of emulsification, cleaning power, or solubilization, depending on the desired outcome. Collecting data from both laboratory and field evaluations allows for a comprehensive understanding of the surfactant’s behavior, leading to informed decisions on its applicability. Moreover, considering environmental compatibility and biodegradability during performance evaluation can ensure that the chosen surfactant not only meets technical requirements but also adheres to sustainability goals.






